Patient-Specific Seizure Onset Detection
Ali Shoeb, Elena Glassman & John Guttag
What and Why
Approximately 1% of the world's population exhibits symptoms of epilepsy [5], a serious disorder of the central nervous system that predisposes those affected to recurrent seizures. A seizure is a sudden breakdown of the neuronal activity of the brain that is clinically manifested by an involuntary alteration in behavior, movement, sensation, or consciousness. These clinical behaviors are preceded and then accompanied by electroencephalographic (EEG) alterations that include discharges of monomorphic (single-frequency) waveforms; polymorphic (multi-frequency) waveforms; spike and sharp wave complexes; or periods of reduced electrocerebral activity [2, 4].
More than 20% of epilepsy patients suffer from seizures that are refractory to medication [6]. For these patients a cerebral resection is an option if the brain region giving rise to seizure activity, the epileptogenic focus, can be identified. Functional imaging modalities, in particular single photon emission computed tomography (SPECT), can be used to localize epileptogenic foci with fine resolution provided that the delay between initiating the scan's protocol, which involves manual intravenous injection of a radiopharmaceutical, and the onset of seizure-associated electroencephalographic alterations is minimized. A SPECT initiated immediately following seizure onset is called an ictal SPECT.
One way to minimize the delay between seizure onset and the start of an ictal SPECT is to have an experienced electroencephalographer continuously monitor the EEG signal over extended periods of time. Doing this is costly, difficult, and mentally taxing and is therefore rarely done. In practice, hospitals inject the radiopharmaceutical following clinical manifestations of a seizure. This often results in large delays because of the subtlety of early clinical signs and the lack of physical proximity of staff responsible for initiating the protocol. On average, injections are started 30-45 seconds after the onset of clinical indications, which could lead to poor localization of the epileptogenic focus and (suspected) downgrading of the clinical value of the procedure.
An automated seizure onset detector can consistently minimize the delay between the onset of electroencephalographic alterations and the initiation of ictal SPECTs. The detector may alert staff to the seizure's onset, or automatically activate a drug infusion pump that delivers the radiopharmaceutical. In collaboration with the Children's Hospital of Boston, we are designing a patient-specific method that can be used to promptly detect the onset of epileptic seizures, and initiate time-sensitive clinical procedures such as ictal SPECT. Our approach is distinctive in that it uses non-invasive EEG, depends on machine learning to introduce patient specificity, and is fully automated.
Additionally, it may be possible to reduce the number of electrodes that the patient must wear. Currently, the detector depends on all 21 electrodes. The elimination of electrodes would make the system more unobtrusive and comfortable to wear for extended periods of time, as well as reduce its computational complexity.
How
We are developing a patient-specific seizure detector to exploit the consistency of both seizure and non-seizure EEG characteristics within patients. This consistency also motivated our treatment of patient-specific seizure detection as a binary classification problem. In such problems, a classifier determines to which of two classes an observation most likely belongs based on a comparison of its features with the learned features of training examples from each of the two classes. In our case, the observation is an EEG signal; its features include the morphology and spatial distribution of waveforms on the scalp; and it is classified as an instance of seizure or non-seizure EEG based on training examples from these classes.
We capture the morphology of EEG waveforms by measuring their energy at different time-scales using a multiresolution wavelet decomposition [3], and we encode their spatial distribution by their placement within the vector of features passed to the classifier, a support-vector machine [1]. This particular classification algorithm was chosen because it is well-suited for classifying nonlinearly separable, high dimensional feature-vectors even when trained on imbalanced training sets. This is crucial given that our detector is expected to recognize waveform morphologies with a certain spatial distribution given an abundance of non-seizure examples, and a far smaller number of seizure training examples.
The block diagram in the figure below presents the architecture of our patient-specific seizure detector. The detector passes 2-second epochs from each of twenty-one bipolar EEG channels through a feature extractor to compute features characterizing the morphology of each channel's waveform. The features extracted from all the channels are grouped into one large feature vector to capture spatial correlations between the channels. This feature vector is then assigned to either the seizure or non-seizure class by way of a support-vector machine trained on previously acquired feature vectors representing patientspecific examples of seizure and non-seizure EEG. Seizure onset is declared only when three consecutive 2-second epochs are classified as members of the seizure class. Requiring seizure activity to last for 6 seconds prior to declaring a seizure event helps avoid false-detections due to short-time, seizure-like activity commonly observed between actual seizures.
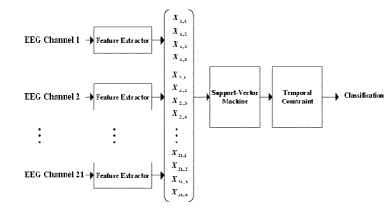
There are many possible algorithms that can be used to determine the smallest possible subset of electrodes with which the detector can still attain sufficient accuracy. For example, greedy and reverse-greedy algorithms involve training the detector on different subsets and picking the subset with which the detector attains the best performance. There is also an algorithm based on discriminability and cross-correlation that does not require the repeated training of the detector; this leaves time for optimization of the detector’s internal parameters, which can also have significant effects on detector performance. Several methods will be implemented with the goal of determining which algorithm is most effective. The results will also indicate how many electrodes can be eliminated for a particular patient.
Progress
Our method was tested on non-invasive EEG recordings from thirty-six pediatric subjects suffering from a variety of seizure types. The recordings were made in a routine clinical environment, so non-seizure activity and common artifacts accompanied seizure EEG. The method exhibited an average latency of 8.0+=3.2 seconds while identifying 131 of 139 seizure events and declared 15 false-detections in 60 hours of clinical EEG. These results demonstrate the high specificity and sensitivity of our seizure detection methodology.
Future
We are currently undertaking a study to quantify the decrease in latency resulting from initiating an ictal SPECT using our method instead of existing hospital protocols.
Research Support
This research was supported by Acer Inc., Delta Electronics Inc., HP Corp., NTT Inc., Nokia Research Center, and Philips Research under the MIT Project Oxygen Partnership, CIMIT, the Center for the Integration of Medicine and Innovative Technology.
References
[1] Nello Cristianini and John Shawe-Taylor. Support Vector Machines and Other Kernel-based Learning Methods. Cambridge University Press, Cambridge, UK, 2000.
[2] ES.Tyner, J.R.Knott, and W.B.Mayer Jr. Fundamentals of EEG Technology: Basic Concepts and Methods, volume Volume 1. Lippincott Wiliams & Wilkins, 1983.
[3] G.Strang and T.Nguyen. Wavelets and Filter Banks. Wellesley-Cambridge Press, 1997.
[4] D. Klass and D. Daly. Current Practice of Clinical Electroencephalography. Raven Press, New York, USA, 1979.
[5] Ilo E. Leppik. Contemporary Diagnosis and Management of the Patient With Epilepsy. Handbooks in Health Care, Newtown, PA, 2000.
[6] The National Society for Epilepsy. Pictures of the future. 2002. http://www.epilepsynse.org.uk.
The Stata Center, Building 32 - 32 Vassar Street - Cambridge, MA 02139 - USA tel:+1-617-253-0073 - publications@csail.mit.edu (Note: On July 1, 2003, the AI Lab and LCS merged to form CSAIL.) |
