Biophysical Models of Neural Computation: MAX and Normalization
Ulf Knoblich & Tomaso Poggio
The Problem
The standard model of object recognition in cortex [9] is composed of a hierarchy of feed-forward layers of neuron-like units, performing one of two basic operations. Either (1) template matching with a Gaussian function to increase feature complexity or (2) nonlinear pooling with a maximum operation to increase translation and scale invariance. However, it is not known how these basic operations could be implemented in cortex.
Motivation
Recently, neurons in different areas of visual cortex of primates and cats have been found to exhibit behavior resembling a soft-max operation [2,3]. Unfortunately the biophysical mechanism underlying these responses is still unknown.
It has been shown that Gaussian-like bell-shaped tuning can be achieved by the combination of normalization and linear summation [5]. Thus, a mechanism for normalization would suffice to explain the bell-shaped tuning that is utilized in the standard model.
New insights into the interplay of excitatory and inhibitory synaptic inputs suggest an important role for the balance of these two. The finding that the ratio of inhibitory and excitatory is actively balanced in the dendrite hints towards the functional significance of this ratio [6]. The crucial importance of timing for the combination of excitatory and inhibitory effects [4] underlines the need for the step from static models to detailed dynamical models of the underlying biophysics of the operations under investigation.
Previous Work
Several circuit models have been proposed to compute either a maximum operation or a normalization or both. [1,5,7,8,10] Although this variety of models exist, it has not been possible to decide which one describes the actual implementation in cortex most accurately. Since none of these models has been implemented in a biophysical framework faithful to neuronal propoerties, it is not entirely clear how closely these mathematical models approximate the actual cortical circuits.
Approach
Using the NEURON simulation environment, we explore dynamical biophysical implementations of the previously presented circuits as well as new models utilizing special biophysical properties of the neurons that can give rise to complex behavior, paying special attention to their dynamic behavior. Investigating these detailed dynamical models then provides a basis for predictions about single neurons and circuits in visual cortex. Using these model predictions, new experiments can be designed to specifically test and distinguish between the different models, determining which model is most accurate.
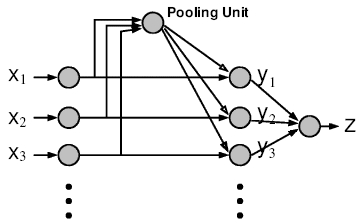
Impact
It is an open question how the two key nonlinear operations (maximum and Gaussian) could be implemented biophysically. Once possible circuits have been modeled biophysically and differential predictions have been tested experimentally, incorporating the most accurate model of the cortical circuits into the standard model of object recognition will then introduce dynamics and establish a much closer correspondence with neurons, including their biophysical properties in cortex.
Future Work
This work is a part of a larger project of continuously validating and enhancing the standard model, which attempts to quantitatively summarize the computational architecture of the ventral pathway with a unifying framework. Future work includes incorporating the electrophysiological data about V1, V2, V4, and IT through close collaborations with experimental groups.
Acknowledgments
This report describes research done at the Center for Biological & Computational Learning, which is in the McGovern Institute for Brain Research at MIT, as well as in the Dept. of Brain & Cognitive Sciences, and which is affiliated with the Computer Sciences & Artificial Intelligence Laboratory (CSAIL).
This research was sponsored by grants from: Office of Naval Research (DARPA) Contract No. MDA972-04-1-0037, Office of Naval Research (DARPA) Contract No. N00014-02-1-0915, National Science Foundation (ITR/SYS) Contract No. IIS-0112991, National Science Foundation (ITR) Contract No. IIS-0209289, National Science Foundation-NIH (CRCNS) Contract No. EIA-0218693, National Science Foundation-NIH (CRCNS) Contract No. EIA-0218506, and National Institutes of Health (Conte) Contract No. 1 P20 MH66239-01A1.
Additional support was provided by: Central Research Institute of Electric Power Industry (CRIEPI), Daimler-Chrysler AG, Compaq/Digital Equipment Corporation, Eastman Kodak Company, Honda R&D Co., Ltd., Industrial Technology Research Institute (ITRI), Komatsu Ltd., Eugene McDermott Foundation, Merrill-Lynch, NEC Fund, Oxygen, Siemens Corporate Research, Inc., Sony, Sumitomo Metal Industries, and Toyota Motor Corporation.
References
[1] M. Carandini and D.J. Heeger. Summation and division by neurons in primate visual cortex. Science, 264:1333--1336, 1994.
[2] I. Lampl et al. Intracellular measurements of spatial integration and the MAX operation in complex cells of the cat primary visual cortex. Journal of Neurophysiology, 92:2704--2713, 2004.
[3] T. Gawne and J. Martin. Responses of primate visual cortical V4 neurons to simultaneously presented stimuli. Journal of Neurophysiology, 88:1128--1135, 2002.
[4] A.T. Gulledge and G.J. Stuart. Excitatory actions of gaba in the cortex. Neuron, 37:299--309, 2003.
[5] M. Kouh and T. Poggio. A general mechanism for cortical tuning: Normalization and synapses can create gaussian-like tuning. MIT AI Memo (In Prep.), 2004.
[6] G. Liu. Local structural balance and functional interaction of excitatory and inhibitory synapses in hippocampal dendrites. Nature Neuroscience, 7(4):373--379, 2004.
[7] T. Poggio and E. Bizzi. Generalization in vision and motor control. Nature, 431:768--774, 2004.
[8] T. Poggio and W. Reichardt. Visual control of orientation behaviour in the Fly. part ii. towards the underlying neural interactions. Q R Bioph., 9(3):377--438, 1976.
[9] M. Riesenhuber and T. Poggio. Hierarchical models of object recognition in cortex. Nature Neuroscience, 2:1019--1025, 1999.
[10] A. Yu, M. Giese, and T. Poggio. Biophysiologically plausible implementations of the maximum operation. Neural Computation, 14(12):2857--2881, 2002.
The Stata Center, Building 32 - 32 Vassar Street - Cambridge, MA 02139 - USA tel:+1-617-253-0073 - publications@csail.mit.edu (Note: On July 1, 2003, the AI Lab and LCS merged to form CSAIL.) |
